- Junior Level (Scroll down for more information)
- Concentration
- surface area of a solid reactant
- concentration or pressure of a reactant
- temperature
- nature of the reactants
- presence/absence of a catalyst.
- active metals with acids, e.g. HCl with zinc
- coal dust with oxygen gas
- grain dust with oxygen gas
- MnO2 in the decomposition of H2O2
- Fe in the manufacture of NH3
- Pt in the conversion of NO and CO to N2 and CO2
- page 466: Method for Measuring Reaction Rates
- page 467: Factors That Affect Reaction Rate
- pages 470-471: Collision Theory and ... Concentration, Surface Area, Nature of Reactants, and Temperature.
- pages 467-468: items 1, 2, 4, 5, and 6
- page 484: items 1 and 2
- pages 486-487: items 1, 2, 3, 4, 5, 6, 9, 12, and 13
- pages 538-541: items 12, 13, 28, 32, 34 and 35
- Why is kindling used to start a fire in a wood stove?
- Consider this chemical equation:
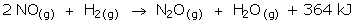
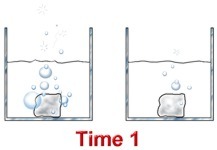
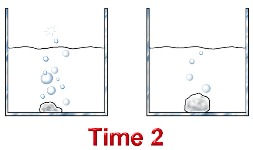
What effect would increasing the temperature from 25°C to 100°C have on the rate of this reaction? Explain. - 10.0 g pieces of zinc are added to 1.00 M and 6.00 M hydrochloric acid solutions at the same time. Assuming there is excess acid in each container, which reaction will proceed faster? Explain.
- In terms of the effect of the nature of reactants on reaction rates, explain the following observations.
- the reaction between perchloric acid and iron is faster than the reaction between nitrous acid and iron.
- sulfur powder reacts slowly with silver, but sulfide ions are rapidly precipitated out of solution in a reaction with silver ions.
- barium reacts with water more readily than calcium
- methane burns more rapidly in air than kerosene (C14H30).
- Cane sugar (sucrose) can be hydrolysed to produce two glucose
molecules. This reaction is very slow.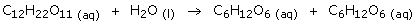
- As the concentration of the reactants increases, the reaction rate increases.
- WHY?
- According to the collision theory, the rate of a reaction is directly proportional to the number of effective collisions per second between the reactant molecules.
- Effective Collisions - the fraction of total collisions that actually result in the formation of the product(s).
- If the concentration of the reactants increases (i.e. particles per given volume) the greater the number of total collisions.
- The greater the frequency of total collisions, the greater the frequency of effective collisions.
- If the frequency of effective collisions increases, so does the reaction rate.
- As the surface area of the reactants increases, the reaction rate increases.
- WHY?
- Increasing the surface area of the reactants results in a higher number of reaction sites.
- Reaction sites - specific sites on molecules at which reactions occur.
- Increasing the number of reaction sites increases the number of total collisions.
- The greater the frequency of total collisions, the greater the frequency of effective collisions.
- If the frequency of effective collisions increases, so does the reaction rate.
- As the temperature of a system increases, the reaction rate increases.
- WHY?
- Temperature (T) - A measure of the average kinetic energy (KEavg) of the particles of a substance.
- Increasing T increases KEavg.
- At higher T, the fraction of molecules with energies greater than the activation energy (Ea) increases.
- Activation Energy (Ea) - the energy level that must be overcome for a reaction to occur.
 | James Clark Maxwell |  | |
Ludwig
Boltzmann |  | ||
| A Maxwell-Boltzmann diagram shows the distribution of molecules at differing kinetic energy levels in a gas sample. At higher temperature, a larger fraction of molecules has kinetic energy equal to or greater than the activation energy (Ea) for the reaction than at lower temperature. In other words, a greater proportion of molecules have enough kinetic energy to participate in the reaction. | |||
CatalystsThe presence of a catalyst increases the reaction rate.
Catalyst - A substance that increases the rate of a reaction but is not consumed in the reaction. It does so by lowering the activation energy (Ea) - The energy level that must be overcome by the reactants in a chemical reaction in order for the reaction to occur) of a reaction. Possible ways of lowering the Ea of a reaction:
Increases the frequency of collisions between the reactant molecules.
Changes the relative orientation of the reactant molecules.
Donates electron density to the reactant molecules.
Reduces the intramolecular bonding within the reactant molecules.
Provides an alternate pathway or mechanism for the reaction.
For equilibrium reactions, both the forward and reverse reaction rates are affected by the catalyst.
- i.e. the Ea for both directions is decreased.
- Therefore, the equilibrium constant (Kc or Kp) - (The product of the concentrations (or partial pressures) of the products of a reaction divided by the product of the
- concentrations (or partial pressures) of the reactants.) is notchanged by the presence of a catalyst.
- The relative concentrations of the reactants and products is not changed.
![[Image]](https://www.chem.tamu.edu/class/majors/tutorialnotefiles/Catalyst.gif)
- Examples of common catalysts:
- Platinum
- Nickel
- Manganese Dioxide (MnO2)
Lesson
Surface Area
Surface area is the exposed matter of a solid substance.Imagine that you are holding a perfect cube of magnesium. The surface area is the sum of the area of all six sides of the cube. The surface area of the cube can be increased by dividing the cube into smaller cubes. Surface area is maximized when a single large cube is crushed to fine powder.
The rate of reaction of a solid substance is related to its surface area. In a reaction between a solid and an aqueous/liquid/gas species, increasing the surface area of the solid-phase reactant increases the number of collisions per second and therefore increases the reaction rate.
In a reaction between magnesium metal and hydrochloric acid, magnesium atoms must collide with the hydrogen ions. When the magnesium atoms form one big lump...
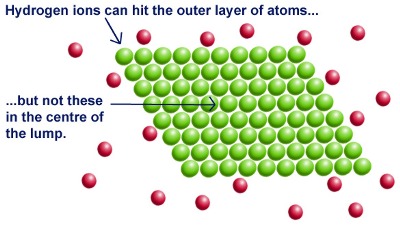
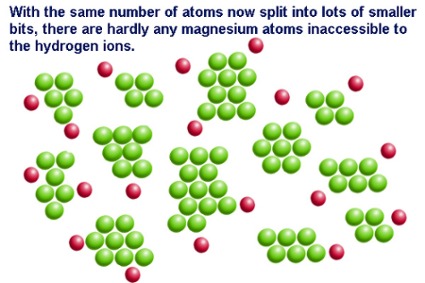
Increasing the surface area of a solid reactant increases the reaction rate.
By increasing surface area, there are more collisions per unit of time. That's why many solids are powdered using a mortar and pestle before being used in a reaction.Examples of other reactions where surface area is important are:
Concentration
The concentration of a substance can be expressed in a variety of ways depending on the nature of a substance. Aqueous solutions typically have their concentrations expressed in mol/L. For example, a solution made by dissolving sodium hydroxide in water has its concentration expressed as moles of NaOH per litre of solution. Gases can also have their concentrations expressed in mol/L.In terms of the collision theory, increasing the concentration of a reactant increases in the number of collisions between the reacting species per second and therefore increases the reaction rate.
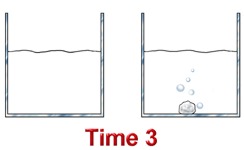
Consider the reaction between hydrochloric acid and zinc metal.
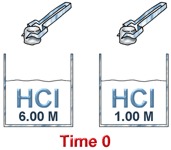
In another, 1.00 mol/L HCl is reacted with 2.00 g of Zn.
Which reaction should occur at the faster rate?
In terms of the collision theory, collisions between zinc atoms and hydrochloric acid are more frequent in the beaker containing 6.0 M HCl - there is more acid per unit of volume.
You can change the concentration of a gas by adding more gas to a fixed volume or by decreasing the volume of the container. Conversely, the concentration of a gas can be decreased by removing (evacuating) a gas from a fixed volume or by increasing the volume of the container.
Pressure
The concentration of a gas is a function of the pressure on the gas. Increasing the pressure of a gas is exactly the same as increasing its concentration. If you have a certain number of gas molecules, you can increase the pressure by forcing them into a smaller volume.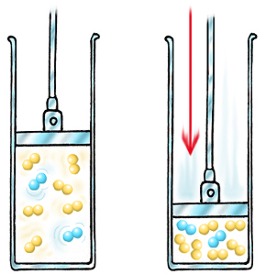
It is important to note however that there are reactions involving gases in which a pressure change does not affect the reaction rate. For this reason, the rates of reactions involving gases have to be determined by experiment.
Also note that solids and liquids are not affected by pressure changes.
Need a good analogy for the effect of concentration on the rate of a chemical reaction?
Temperature
With the exception of some precipitation reactions involving ionic compounds in solution, just about all chemical reactions take place at a faster rate at higher temperatures. The question is why?
 Temperature (in Kelvin degrees) is proportional to the kinetic energy of the particles in a substance. For example, if the Kelvin temperature of a substance is doubled, then the average kinetic energy of the particles in that substance is doubled.
Temperature (in Kelvin degrees) is proportional to the kinetic energy of the particles in a substance. For example, if the Kelvin temperature of a substance is doubled, then the average kinetic energy of the particles in that substance is doubled.At higher temperatures, particles collide more frequently and with greater intensity.
Here's an analogy.
Imagine that you are baby-sitting a bunch of 6 year olds. You put them in a yard and you let them run around. Every now and then a couple of kids will run into each other. Now imagine that you decide to feed them some sugar. What happens? They run around faster and of course there are many more collisions. Not only that, the collisions are likely to be a lot harder/more intense.Now, let's look at the effect graphically. Recall that in any sample of matter (the example we used previously was a gas), individual particles have different kinetic energies. Some are moving fast some are moving slowly, and most are moving at some intermediate speed.
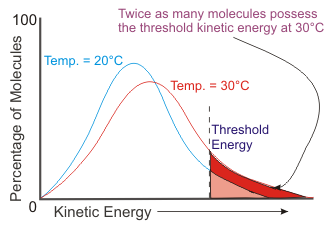
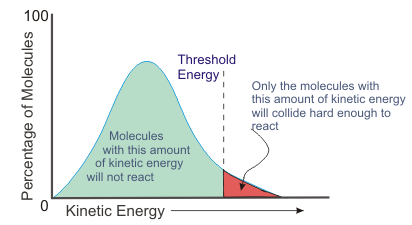
Now consider the relationship between threshold kinetic energy and activation energy. Threshold kinetic energy is the minimum amount of energy required for colliding particles to react - it is the equivalent of activation energy or the minimum potential energy gain required to form an activated complex.
As you can see on the graph, a small increase in temperature can double the number of molecules with the threshold kinetic energy.
A general rule is that a 10°C temperature increase can double a reaction rate. It turns out that the increase in the reaction rate is mainly a function of the more intense collisions. Increased collision frequency is not as significant a factor.
Nature of Reactants
Individual properties of substances also affect reaction rates. The scope of these properties is broad and there are few generalizations that you can apply consistently. Some of the properties in this category are state of matter, molecular size, bond type and bond strength.State of Matter
Gases tend to react faster than solids or liquids: It takes energy to separate particles from each other. In order to burn candle wax, the solid wax has to be melted and then vaporized before it reacts with oxygen. Methane gas is already in the gas state so it burns faster than wax.Aqueous ions tend to react faster than species in other states of matter: Solid lead(II) nitrate will react with solid potassium iodide, but the reaction is really, really slow. That's because the ionic bonding in each reactant is strong and the ions in each compound are hard to separate from each other. When aqueous solutions of these compounds are mixed, the formation of lead(II) iodide is rapid. In aqueous solutions, the ions of each compound are dissociated. When the two the solutions are mixed together, all that is required for a reaction to occur is contact between the lead(II) ions and the iodide ions.fast
slow
Bond Type
Reactions involving ionic species tend to proceed faster than reactions involving molecular compounds.Bond Strength
Reactions involving the breaking of weaker bonds proceed faster than reactions involving the breaking of stronger bonds. For example, double carbon to carbon bonds are stronger than single C-C bonds.Number of Bonds/Molecular Size
Reactions involving the breaking of fewer bonds per reactant proceed faster than those involving the breaking of a larger number of bonds per reactant.The simple ion Fe2+ reacts faster than oxalate (OOCCOO2-).
Kerosene burns more slowly than methane because there are more bonds to be broken per molecule of kerosene than there are per molecule of methane. Kerosene is a larger molecule
fast
slow
Catalyst
A catalyst is a species that speeds up a chemical reaction without being chemically changed upon completion of the reaction. In other words, the mass of a catalyst is the same before and after a reaction occurs.Common examples of catalysts include:
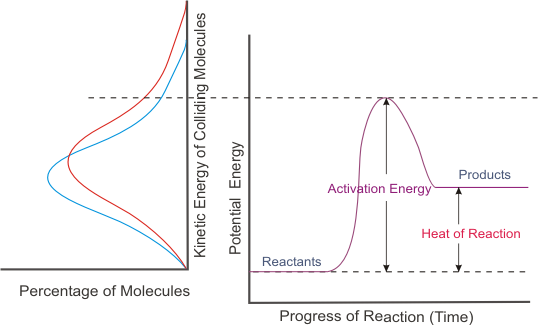
Also recall that activation energy corresponds to threshold energy.
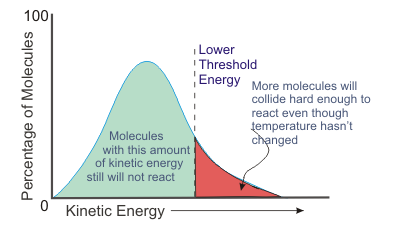
The number of successful collisions per unit of time be increased by lowering the threshold energy (or in terms of potential energy, lowering the activation energy).
A catalyst provides an alternative pathway for the reaction - a pathway that has a lower activation energy. Be careful how you say it.
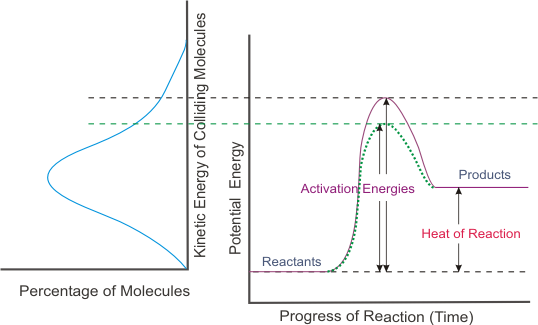
The catalyzed pathway (shown as a dotted green line above) has lower activation energy.
Relating this back to the kinetic energy diagram, you see that more particles will have sufficient kinetic energy to react. In other words, the addition of the catalyst increases the reaction rate.
You'll explore the effect of a catalyst further on reaction rate in the next lesson.